Gene expression arrays and immunohistochemical phenotypes studies classified breast cancer in three distinct subtypes: basal, HER2/neu and luminal that are associated with different clinical outcomes. In 141 patients with operable breast cancer included in several phase III adjuvant therapy trials, immunohistochemical staining was performed. A basal phenotype was defined by negative estrogen and progesterone receptor and positive cytokeratin 5/6 or EGFR immunoreactivity. HER2/neu phenotype as positive c-erb B2 by HercepTest™ and luminal phenotype by positive estrogen receptors, progesterone receptors and CK 7/8 and negative HER-2 were defined respectively. Survival curves were calculated by the Kaplan-Meier method. The differences between survivals were estimated using the log rank test.
Prognostic value and response to chemotherapy of immunohistochemical phenotypes of 141 operable breast cancer patients included in phase III trials of adjuvant therapy
Rafael Trujillo 1, E Gallego 3, A Márquez 2, N Ribelles 2, D Perez 1, C Quero 2, D Olmos 1, L Vicioso 3, A Rueda 1, E Alba 1
1. Hospital Costa del Sol, Medical Oncology Department, MARBELLA, SPAIN
2. Hospital Universitario Virgen de la Victoria, Medical Oncology Department, MALAGA, SPAIN
3. Hospital Universitario Virgen de la Victoria, Pathology Department, MALAGA, SPAIN
Abstract:
Background: Gene expression arrays and immunohistochemical phenotypes studies classified breast cancer in three distinct subtypes: basal, HER2/neu and luminal that are associated with different clinical outcomes.
Methods: In 141 patients with operable breast cancer included in several phase III adjuvant therapy trials, immunohistochemical staining was performed. A basal phenotype was defined by negative estrogen and progesterone receptor and positive cytokeratin 5/6 or EGFR immunoreactivity. HER2/neu phenotype as positive c-erb B2 by HercepTest™ and luminal phenotype by positive estrogen receptors, progesterone receptors and CK 7/8 and negative HER-2 were defined respectively. Survival curves were calculated by the Kaplan-Meier method. The differences between survivals were estimated using the log rank test.
Results: The median follow-up period was 52 months (range 1-103 months). During this period, 13.8% patients died from breast cancer and 27.7% patients relapsed. At the time of the primary diagnosis 10.4% of the patients had lymph node negative disease, 50.8% patients received standard chemotherapy and 7.7% Trastuzumab. Positivity for luminal phenotype was 65.2%, basal phenotype 9.9%, HER-2 phenotype 8.5% and 16.3% were null/no expression. Median disease free survival for basal phenotype was 24 months, but for luminal phenotype and HER-2 phenotypes has not been reached. DFS at 5 years were basal phenotype: 19%, luminal phenotype: 63% and HER-2: 56%. Detectable basal phenotype was highly significantly associated with a worse DFS compared with the presence of a non basal phenotype (p=0.0001). Multivariate analyses estimated that the prognostic effect of basal phenotype was independent of lymph node, stage and tumor size (HR 0.12; 95% CI 0.05-0.2; p<0.05). In the group of patients who received standard-based adjuvant chemotherapy, disease free survival was found to be significantly shorter in the basal phenotype (p<0.05).
Conclusions: We found that expression of basal phenotype was associated with poor prognostic in the context of randomized phase III trials. Standard adjuvant chemotherapy seems to be less effective in these tumors and new therapeutic approaches are indicated.
Correspondence to: Dr Rafael Trujillo. Hospital Costa del Sol, Medical Oncology Department, Autovía A-7 Km 187. 29603 Marbella , SPAIN.
Keywords: Breast cancer, genetics; Microarrays; Chemotherapy, adjuvant ; Breast cancer, prognostic factors
Introduction
Breast cancer is one of the most common malignancies in women (1). Currently, the most important prognostic factors are nodal status, tumor size, status of hormone receptor, and histological grade. Nevertheless numerous other clinicopathologic factors and novel molecular markers have been investigated to improve the prediction of clinical outcome (2). Individual prognostic factors provide limited information on the biology of the disease because of the heterogeneity of breast cancers as well as the interrelationship among prognostic markers.
Molecular classification based on gene expression patterns from complementary DNA (cDNA) microarrays has recently been applied for breast cancer (3,36,39,40,41)showing to be a powerful tool to predict tumors behavior. Studies focusing on breast cancer have been published showing that gene expression profiling distinguishes groups of patients representing specific tumor subtypes and/or prognosis (4,35,36,37,39,40,41). It has been shown that using the gene expression profile of breast cancer, prognosis can be more accurately predicted than by clinical variables only (5,35,36,37,39,40,41),and different subtypes of invasive breast carcinomas including luminal/estrogen receptor (ER)+, normal breast–like, HER2/neu+, and basal-like subtypes can be distinguished (6,36,37,39,40,41). Among these, the basal-like and HER2/neu+ subtypes are associated with poorer clinical outcomes than other types in Western countries.
Tumors expressing basal epithelium cytokeratins (CK5/14/17) constitute a subgroup distinct from basal cytokeratin-negative luminal epithelium-like tumors. Basal cytokeratin-positive tumors are characterized by hormone receptor negativity, a high tumor grade, and a high proliferation activity (7). Gene expression microarray studies have also distinguished the "basal-like" tumors from the other subtypes of tumors, and have associated the basal-like group with adverse patient survival (8). Different immunohistochemical markers have been used by different investigators to identify basal-like differentiation, but there is no a universally agreed-upon criterion or set of markers that define this subtype of breast cancer. Nielsen et al. (8) developed a panel for identifying basal-like breast cancers based on a comparison of immunohistochemical profiles. Using their definition, basal-like carcinomas are negative for estrogen receptors and HER2, in addition to being positive for either cytokeratin 5/6 or epidermal growth factor receptor (EGFR). Others have suggested that expression of high molecular weight cytokeratins alone (including CK5, CK14, and CK17) could be used (11). By immunohistochemistry, the expression of CK5/14/17 can be detected in 10% of breast tumors (9). The overwhelming majority of breast cancers are characterized by the expression of CK8/18 only, the predominant cytokeratins of the glandular epithelium lining the lumen of the breast ducts (10). Therefore, in most immunohistochemical studies, the classification of breast cancers into "basal phenotype" and "luminal phenotype" subgroups refers to the presence or absence of CK5/14/17 in the tumor cells, respectively (11), because CK8/18 can be detected in all sporadic CK5/14-positive breast tumors. It has been suggested that, until these criteria are developed, staining for estrogen receptors, progesterone receptors, and HER2 would correctly classify the majority of basal-like breast cancers (46). Currently, three large molecular subgroups have been proposed: HER2-overexpressing/Estrogen receptor (ER)-negative tumors, basal-like and luminal-like breast cancers.
In addition, the study of these molecular subtypes of breast cancer might offer some predictive information about treatment efficacy. Studies have investigated the predictive value of HER2 status for the efficacy of anthracyclines (12,13,14). In two out of four studies, Her2 expression was found to be predictive for the benefit of anthracyclines in terms of disease-free survival (13,14).Several studies have reported a good correlation between the molecular subclassification (determined by DNA microarray) and the efficacy of postoperative and preoperative anthracycline-based chemotherapy (12,13,14,38). Recently, Conforti et al (42), have published that estrogen receptors expression was a strong and independent predictive biomarker for the benefit of adjuvant chemotherapy. In this study, the authors also found that the P value for interaction between HER2 expression and benefit of chemotherapy was 0.37, and concluded that HER2 did not add predictive information to that provided by the estrogen receptors status. Furthermore, while the molecular subclassification was predictive for the efficacy of chemotherapy (test for interaction, P = 0.01), its determination did not add significant information to the predictive value provided by the estrogen receptors status (P = 0.32).
In the present study, in a well-characterized and followed series of primary breast cancer that entered in randomized phase III trials, we determined the immunohistochemically expression pattern in 141 breast tumors from clinical adjuvant therapy randomized trials. The results were correlated with the biological and clinicopathologic tumor characteristics and patient survival.
Patients and methods
Patients
One hundred and forty one cases of primary operable invasive breast carcinoma from patients that entered in randomized phase III trials were analyzed. Patients have been followed up at 3-month intervals initially, then 6-monthly, then annually. At the time of resection all tumours are managed in a standard fashion, incised and fixed immediately.
Tissue array and immunostaining
A basal phenotype was defined by negative estrogen and progesterone receptor and positive cytokeratin 5/6 or EGFR immunoreactivity. HER2/neu phenotype as positive c-erb B2 by HercepTest™ and luminal phenotype by positive estrogen receptors, progesterone receptors and CK 7/8 and negative HER-2 were defined respectively.
Immunohistochemical staining was performed on 3μm sections of paraffin blocks containing tissue-arrays of tumour tissue. To construct these blocks, two representative areas from the tumor were selected from a hematoxylin-eosin stained section of a donor block. Core cylinders (0.4 mm diameter) were punched from each of them and deposited into two separate recipient paraffin blocks. The sections were microwaved in citrate buffer, pH 6 for 20 min, to epitope retrieval. Immunohistochemical test was carried out in an autoimmunostainer (Tech Mate Horizon, Dako, Copenhagen, Denmark), using the Dako Real EnVision method to detect the antibody.
The following antibodies were used: estrogen receptor (clone 1D5, Dako, diluted 1:35), progesterone receptor (clonePgR636, Dako, diluted 1:50), c-erb B2 (HerceptestTM, Dako) CK 5/6 (clone D5/16B4, Boehringer Biochemica, diluted 1:50), CK 7/8 (clone CAM 5.2, Becton Dickinson, prediluted), CK 14 (clone LL002, Novocastra, diluted 1:100), CK 18 (clone DC10, Dako, diluted 1:25), CK 19 (clone Bck 108, Dako, diluted 1:100) and SMA (clone 1A4, Dako, diluted 1:100). Immunohistochemistry of EGFR (EGFR pharmaDx TM for autostainer, Dako) was made on full tissue sections.
For estrogen receptors and progesterone receptors immunoreactivity, the cut-off value of 10% was used to divide cases into negative and positive groups. C-erb B2 expression was scored with the Dako scale (HercepTest kit scoring guidelines) .EGFR pharmDx test results were evaluated as positive or negative, using membrane staining as the evaluable structure. The gradation of staining depended on the intensity of staining (0 no staining, 1 weak, 2 moderate, and 3 strong staining) and the percentage of stained cells. Positivity for EGFR expression was defined as any membrane staining above background level, whether or not completely circumferential in, all most, 1% of tumor cells. Absence of staining was reported as negative. Luminal markers CK 7/8, 18 and 19, were identified as negative (weak or negative expression) or positive (moderate and strong positive expression). For basal markers, CK 5/6, CK 14 and SMA, positivity was defined as detection of any invasive malignant cells positive.
Statistical analysis.
The primary end point was DFS, defined as the time between the date of randomization and the date of the last follow-up or the date of the first event: locoregional recurrence, distant metastasis, contralateral breast tumor or death.
Association between the immunoreactivity with the various antibodies used and different clinicopathological parameters was evaluated by chi-squared test. A p-value of <0.05 was considered to reflect a significant correlation. Survival curves were calculated by the Kaplan-Meier method. The differences between survivals were estimated using the log rank test. Multivariate Cox regression analysis was used to evaluate any independent prognostic effect of the variables on DFS. Two-sided P values of P < 0.05, and of <0.01 for the interaction tests, were considered statistically significant. All analyses were carried out using SPSS 11.0 statistical package.
Results
Patient’s characteristic and clinical outcomes
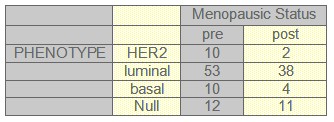
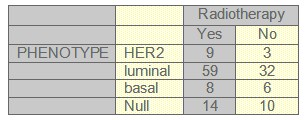
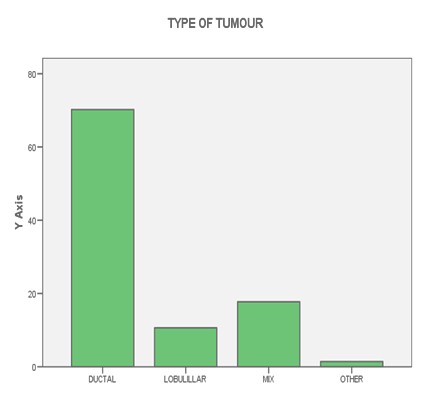
Complete clinical follow-up information was available for 141 patients. The median follow-up was 52 months (range 1-103 months). During this period, 13.8% patients died from breast cancer and 27.7% patients relapsed. Fifty-six patients were postmenopausal, and at the time of the primary diagnosis 10.4% had lymph node negative disease. 50.8% patients received standard chemotherapy, 7.7% Trastuzumab, 62.3% radiotherapy and 61% patients received hormonotherapy. Other patients characteristic can be seen on tables (1-2-3-4-5) and figures (1-2)
TABLE 1: Histology and phenotype
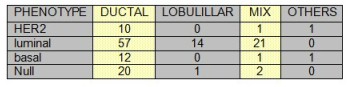
TABLE 2: Number of lymph nodes and phenotype
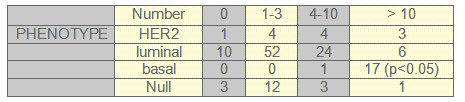
TABLE 3: Menopausic Status and phenotype
TABLE 4: Radiotherapy and phenotype
TABLE 5: Histology grade and phenotype
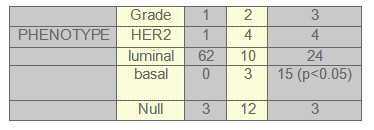
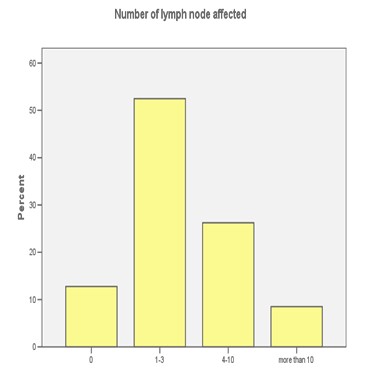
FIGURE 1: LYMPH NODES
FIGURE 2: HISTOLOGY
Phenotypes markers expression and Phenotypes distribution:
141 tumors were available for CK 7/8, 18 and 19, CK5/6, 14 and SMA analyses respectively. A very high proportion of cases, 65.2%, demonstrated positivity for the luminal cytokeratins 7/8, 18 and 19. In contrast, a lower proportion, 9.9%, showed expression of the basal markers. There was a highly significant inverse correlation between the luminal (CK 7/8, CK18 and CK19) and the basal (CK 5/6, CK14 and SMA) immunoprofiles (p < 0.05).
We found that expression of luminal markers was associated with good prognostic tumour characteristics: 62 patients were grade 1 and 52 patients had only one to three lymph nodes affected. These findings are in contrast to the expression of basal markers, which was associated with poor prognostic features: 17 patients had more than 10 lymph nodes affected and 15 patients were grade 3, both of them were statistical significant (p<0.05).
Luminal phenotype was present in 65.2% of the cases, basal phenotype in 9.9% and HER-2 phenotype in 8.5%. In 16.3% of the tumors the phenotype were considered null/no expression.
Phenotypes and survival.
Median DFS for basal phenotype was 24 months, and for luminal and HER-2 phenotypes the median disease DFS was not reached. Five years disease free survival was as follows: basal phenotype 19%, luminal phenotype 63% and HER-2 56%. The DFS analysis by phenotypes subtypes has not been reached statistical significance. Nevertheless, when the same analysis for basal phenotype compared with non basal phenotype was done, the difference was highly significant showing that the presence of a detectable basal phenotype was associated with a worse disease free survival (Figure 8) (p= 0.0001).
Figure 8. Disease free survival (months).
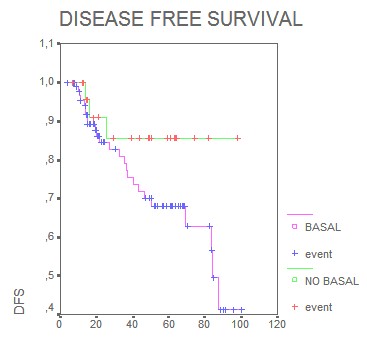
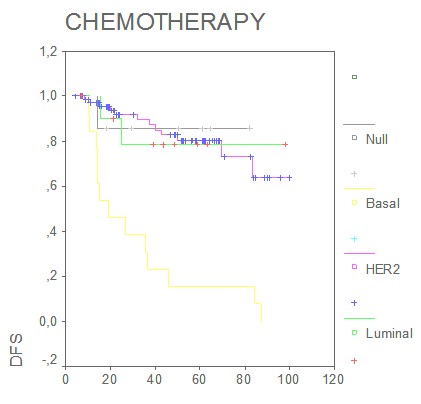
Interestingly, multivariate Cox regression analyses estimated that the prognostic effect of basal phenotype in relation to disease free survival was independent of lymph node, stage and tumor size (HR 0.12; 95% CI 0.05-0.2; p<0.05) The analysis of the possible predictive value of tumor phenotypes found that in the group of patients who received standard-based adjuvant chemotherapy, DFS was significantly shorter in the basal phenotype, (p= 0.001) (Figure 9, months).
Figure 9:
Discussion
In the last few years the studies of gene expression patterns or inmonohistochemical profiles focusing in breast cancer (43,44,45,46,47), have been shown that is possible to predict the behavior of the disease more accurately than by classical prognostic factors only.
Recent cDNA gene expression analysis and immunohistochemical studies have proposed two distinguishable groups with luminal and basal phenotypes that have different cytogenetic alterations and protein expression patterns (22,23,36,37,39,40)
Our results demonstrate on a well-characterized and followed series of primary breast cancer that a high proportion of 65.2% invasive breast carcinomas express only the luminal epithelial cell cytokeratins and have a pure luminal cell phenotype. Exclusive expression of the basal epithelial markers was restricted to a very small subset. Previous studies have also recorded that the luminal CKs 7, 8, 18 and 19 are predominantly expressed in breast cancer (15), like in our study. Expression of the basal markers CK 5/6, CK14 and SMA has been reported in invasive breast carcinoma, ranging from 4% to 16% of cases (16). This is in agreement with our study, wherein the expression of the basal phenotype was positive in 9.9% of the cases.
On studying the association between the expression of these markers with different clinical and pathological parameters, we found that expression of luminal markers were associated with good prognostic tumor characteristics and outcome, in contrast to the expression of basal markers, which was associated with poor prognostic features and behavior. These findings are in accordance with previous studies where an inverse association between CK 8, 18 expression and tumor grade, recurrence rate and estrogen receptors negative status has been reported (17, 43). They also support previous data which have shown that highly metastatic cell lines are associated with loss of CK 18 expression (18).
In our series the presence of a detectable basal phenotype was highly significantly associated with a worse prognosis, so that the DFS at 5 years were nearly three times greater in the non basal group than in the basal one.Similar results have been reported by other authors. Perou et al (23) found that women with basal-like breast cancers had shorter relapse-free survival times than women with other types of breast cancer. Basal-like breast cancers also have a tendency toward visceral (versus bone) metastasis (24). In an analysis of 49 patients with basal-like breast cancer and 49 matched controls, Banerjee et al. (25) found that patients with basal-like breast cancer had significantly shorter disease free and overall survival times than women with other tumors, but basal-like status was not a significant independent prognostic variable in the multivariable analysis.
Studies on patients with basal-like breast cancers have been limited by small sample sizes and short follow-up times. To some extent, this is due to the basal-like phenotype is based on immunohistochemical staining of tumor slides using anti-keratin antibodies, and these are not yet in general clinical use. However, the ‘‘basal-like’’ category of tumors is composed almost entirely of ‘‘triple-negative’’ breast cancers [i.e., tumors that are negative for estrogen receptors, progesterone receptors (PR), and HER2]. It is therefore possible to classify with accuracy the majority of basal-like breast cancers (and non– basal-like breast cancers) using these three standard immunohistochemical markers. Dent et al published very recently (26) a large series of triple-negative breast cancers derived from a single institution with long-term follow-up. They were able to show that the triple-negative category of breast cancers exhibits a distinct pattern of recurrence. This pattern is characterized by a rapidly rising rate in the first 2 years following diagnosis, a peak at 2 to 3 years followed by a decline in recurrence risk over the next 5 years, and a very low risk of recurrence thereafter. Unlike women with other types of breast cancers, the great majority of women with triple-negative cancers who had no evidence of progression after 8 years did not recur thereafter.
Expression of the luminal markers was significantly related to longer DFS in our series, in particular, patients with high or moderate expression showed better DFS than those cases with low or no expression of these luminal markers. The converse was observed in tumors that labeled with the basal markers, where positive cases were associated with poor outcome particularly with CK 5/6 expression, which proved to be an independent prognostic predictor for DFS. Previous studies have reported that CK 8 is associated with better overall survival and is an independent prognostic indicator of DFS, and that CK 18 expression is an independent prognostic factor in predicting DFS In contrast, a significant association has been reported between poor overall survival and expression of the basal markers, CK 5/6 and 17, and these markers also had an independent prognostic impact in patients without nodal metastasis. Positivity for both CK 5/6 and SMA was reciprocally related to age, supporting the finding that breast cancer in younger women is more aggressive, with lower hormone receptor levels, higher proliferation and a worse prognosis compared with those in women of older age (19).
On studying the expression of basal markers in different morphological tumor types, we found that tumors of special type were either absolutely negative or expressed these markers in a small proportion of cases. We found that invasive grade 3 carcinomas of no special type had a basal phenotype and were estrogen receptors negative. The same finding has been reported in a previous study (20).These findings are in keeping with two previous reports that reported a high prevalence of the basal phenotype in ductal carcinoma with extensive necrosis and their aggressive behavior (21). Overall, tumors expressing the basal phenotype were more often grade 3 tumors and estrogen receptors negative with poor outcome. A previous genetic study on grade 3 carcinoma of no special type with basal phenotype identified specific genetic alterations and the aggressive nature of that subset (22).
It has been reported previously that molecular subclassification of breast cancer was associated with efficacy of postoperative and preoperative chemotherapy (12,13,14,38). In our study, the group of patients who received standard-based adjuvant chemotherapy, DFS was found to be significantly shorter in the basal phenotype. Nearly 8% of the patients that were HER2 positive received trastuzumab therapy, and this fact could have influenced the good prognostic of HER2 phenotype in our series.
Despite differences in taxonomy, there is a consistent trend across all studies confirming the relatively poor prognosis of the triple-negative or basal-like breast cancer subgroup (27). The majorities of triple-negative breast cancer tumors overexpress the EGFR (16) and might be candidates for anti-EGFR and/or anti–vascular endothelial growth factor therapies (28). Tumors in women with BRCA1 germ-line mutations have similarities to basal-like breast cancers (29). In vitro chemosensitivity studies have found that human cells lacking BRCA1 may be sensitive to cisplatin and to other drugs that cause double-strand breaks in DNA (30). Thus, agents such as cisplatin or carboplatin may prove to be effective treatments for the basal like group.
Despite the complexity of expression of the markers used in the present study, we were able to identify four profiles: luminal, HER2, basal and null/no expression. This supports the finding of studies that have, similarly, reported cases of breast cancer with null/no expression markers (31,32,33).Two previous studies have reported cases of breast cancer which were negative for both the luminal and basal markers (33,34). Both studies used frozen section material in which immunoreactivity was optimally preserved. One could argue that cases having no demonstrable phenotype may be a consequence of loss of reaction due to differences in tissue handling. However, all cases in our study were handled in a similar way and optimally fixed in formalin.
In conclusion, we found that expression of basal phenotype was associated with poor prognostic in the context of randomized phase III trials. The high rates of distal recurrence and the low incident of local recurrence, suggest that these patients have a tendency to develop visceral metastases early in the course of their disease. Standard adjuvant chemotherapy seems to be less effective in these tumors and new therapeutic approaches are indicated. The majorities of triple-negative breast cancer tumors overexpress the EGFR (16) and might be candidates for anti-EGFR and/or anti–vascular endothelial growth factor therapies (28). In this respect, different clinical trials are now under way, like GEICAM/2006-03 clinical trial were neoadyuvant based carboplatin chemotherapy is used in basal phenotype. This trial and others will answer these questions in the near future.
Bibliography
A. Jemal, R. Siegel and E. Ward et al., Cancer statistics, 2006, CA Cancer J Clin 56 (2006), pp. 106–130.
1. E.R. Fisher, J. Costantino, B. Fisher and C. Redmond, Pathologic findings from the National Surgical Adjuvant Breast Project (Protocol 4). Discriminants for 15-year survival. National Surgical Adjuvant Breast and Bowel Project Investigators, Cancer 71 (1993), pp. 2141–2150.
2. Van de Vijver MJ, He YD, van 't Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002; 347:1999-2009.
3. Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869–74.
4. Wang Y, Klijn JG, Zhang Y, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 2005;365:671–9.
5. Sotiriou C, Neo SY, McShane LM, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003 Sep 2;100(18):10393-8.
6. Laakso M, Loman N, Borg Å, Isola J. Cytokeratin 5/14-positive breast cancer: true basal phenotype confined to BRCA1 tumors. Mod Pathol 2005;18:1321–8.
7. Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004;10:5367–74.
8. Otterbach F, Bànkfalvi À, Berhner S, Decker T, Krech R, Boecker W. Cytokeratin 5/6 immunohistochemistry assists the differential diagnosis of atypical proliferations of the breast. Histopathology 2000;37:232–40
9. Boecker W, Moll R, Poremba C, et al. Common adult stem cells in the human breast give rise to glandular and myoepithelial cell lineages: a new cell biological concept. Lab Invest 2002;82:737–45.
10. Abd El-Rehim DM, Pinder SE, Paish CE, et al. Expression of luminal and basal cytokeratins in human breast cancer. J Pathol 2004;203:661–71.
11. Paik S, Bryant J, Park C, et al. erbB-2 and response to doxorubicin in patients with axillary lymph node-positive, hormone receptor-negative breast cancer. J Natl Cancer Inst (1998) 90:1361–1370.
12. Pritchard KI, Shepherd LE, O'Malley FP, et al. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med (2006) 354:2103–2111.
13. Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res (2005) 11:5678–5685.
14. Rejthar A, Nenutil R. The intermediate filaments and prognostically oriented morphological classification in ductal breast carcinoma. Neoplasma 1997; 44: 370-373.
15. Van de Rijn M, Perou CM, Tibshirani R, et al. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol 2002; 161: 1991-1996.
16. Brotherick I, Robson CN, Browell DA, et al. Cytokeratin expression in breast cancer: phenotypic changes associated with disease progression. Cytometry 1998; 32: 301-308.
17. Schaller G, Fuchs I, Pritze W, et al. Elevated keratin 18 protein expression indicates a favorable prognosis in patients with breast cancer. Clin Cancer Res 1996; 2: 1879-1885.
18. Ferno M, Borg A, Johansson U, et al. Estrogen and progesterone receptor analyses in more than 4000 human breast cancer samples: a study with special reference to age at diagnosis and stability of analyses. Southern Swedish Breast Cancer Study Group. Acta Oncol 1990; 29: 129-135.
19. Tot T. The cytokeratin profile of medullary carcinoma of the breast. Histopathology 2000; 37: 175-181.
20. Tsuda H, Takarabe T, Hasegawa F, Fukutomi T, Hirohashi S. Large, central acellular zones indicating myoepithelial tumor differentiation in high-grade invasive ductal carcinomas as markers of predisposition to lung and brain metastases. Am J Surg Pathol 2000; 24: 197-202.
21. Korsching E, Packeisen J, Agelopoulos K, et al. Cytogenetic alterations and cytokeratin expression patterns in breast cancer: integrating a new model of breast differentiation into cytogenetic pathways of breast carcinogenesis. Lab Invest 2002; 82: 1525-1533.
22. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52.
23. Rodriguez-Pinilla SM, Sarrio D, Honrado E, et al. Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res 2006;12:1533-9.
24. Banerjee S, Reis-Filho JS, Ashley S, et al. Basal-like breast carcinomas: clinical outcome and response to chemotherapy. J Clin Pathol 2006;59:729-35.
25. Dent R, Trudeau M, Pritchard K,et al, Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence Clin Cancer Res 2007;13:4429-34.
26. Abd El-Rehim DM, Ball G, Pinder SE, et al. Highthrough put protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses.Int J Cancer 2005;116:340-50.
27. Siziopikou KP, Cobleigh M. The basal subtype of breast carcinomas may represent the group of breast tumours that could benefit from EGFR-targeted therapies. Breast 2007;16:104-7.
28. Narod SA, FoulkesWD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer 2004;4:665-76.
29. Ratanaphan A, Canyuk B,Wasiksiri S, MahasawatP. In vitro platination of human breast cancer suppressor gene (BRCA1) by the anticancer drug carboplatin. Biochim Biophys Acta 2005;1725:145-51.
30. Nagle RB, Bocker W, Davis JR, et al. Characterization of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. J Histochem Cytochem 1986; 34: 869-881.
31. Malzahn K, Mitze M, Thoenes M, Moll R. Biological and prognostic significance of stratified epithelial cytokeratins in infiltrating ductal breast carcinomas. Virchows Arch 1998; 433: 119-129.
32. Wetzels RH, Kuijpers HJ, Lane EB, et al. Basal cell-specific and hyperproliferation-related keratins in human breast cancer. Am J Pathol 1991; 138: 751-763.
33. Dairkee SH, Puett L, Hackett AJ. Expression of basal and luminal epithelium-specific keratins in normal, benign, and malignant breast tissue. J Natl Cancer Inst 1988; 80: 691-695.
34. Weigelt B, Glas AM, Wessels LF et al. Gene expression profiles of primary breast tumors maintained in distant metastases.Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):15901-5.
35. Van´t Veer L, Dai H, van de Vijver Mj et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002 Jan 31;415(6871):530-6.
36. Foekens JA, Atkins D, Zhang Y, et al. Multicenter validation of a gene expression-based prognostic signature in lymph node-negative primary breast cancer. J Clin Oncol. 2006 Apr 10;24(11):1665-71.
37. Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006 Aug 10;24(23):3726-34.
38. Liu R, Wang X, Chen GY, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007 Jan 18;356(3):217-26.
39. Fan C, Oh DS, Wessels L, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med. 2006 Aug 10;355(6):560-9.
40. Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8418-23.
41. Conforti R, Boulet T, Tomasic G, et al. Breast cancer molecular subclassification and estrogen receptor expression to predict efficacy of adjuvant anthracyclines-based chemotherapy: a biomarker study from two randomized trials. Annals of Oncology.2007. Sept 18: 1477–1483.
42. de Mesquita JM Bueno, van Harten WH, Retel VP, et al. Use of a 70-gene signature to predict prognosis of patients with node-negative breast cancer: a prospective community-based study (RASTER). Lancet Oncol 2007; 8: 1079-1087.
43. Glas AM, Floore A, Delahaye LJ, et al. Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics 2006; 7: 278 Cowell JK, Hawthorn L. The application of microarray technology to the analysis of the cancer genome. Curr Mol Med 2007; 7:103–120..
44. Buyse M, Loi S, van't Veer L, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst 2006; 98: 1183-1192.
45. Bertucci F, Finetti P, Cervera N, Maraninchi D, Viens P, Birnbaum D. Gene expression profiling and clinical outcome in breast cancer. OMICS 2006; 10: 429-443.
46. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006; 295:2492–2502.
47. Hu Z, Fan C, Oh DS, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics 2006; 7:96.