Response to chemotherapy of immunohistochemical phenotypes of breast cancer patients.2
The following antibodies were used: estrogen receptor (clone 1D5, Dako, diluted 1:35), progesterone receptor (clonePgR636, Dako, diluted 1:50), c-erb B2 (HerceptestTM, Dako) CK 5/6 (clone D5/16B4, Boehringer Biochemica, diluted 1:50), CK 7/8 (clone CAM 5.2, Becton Dickinson, prediluted), CK 14 (clone LL002, Novocastra, diluted 1:100), CK 18 (clone DC10, Dako, diluted 1:25), CK 19 (clone Bck 108, Dako, diluted 1:100) and SMA (clone 1A4, Dako, diluted 1:100). Immunohistochemistry of EGFR (EGFR pharmaDx TM for autostainer, Dako) was made on full tissue sections.
For estrogen receptors and progesterone receptors immunoreactivity, the cut-off value of 10% was used to divide cases into negative and positive groups. C-erb B2 expression was scored with the Dako scale (HercepTest kit scoring guidelines) .EGFR pharmDx test results were evaluated as positive or negative, using membrane staining as the evaluable structure. The gradation of staining depended on the intensity of staining (0 no staining, 1 weak, 2 moderate, and 3 strong staining) and the percentage of stained cells. Positivity for EGFR expression was defined as any membrane staining above background level, whether or not completely circumferential in, all most, 1% of tumor cells. Absence of staining was reported as negative. Luminal markers CK 7/8, 18 and 19, were identified as negative (weak or negative expression) or positive (moderate and strong positive expression). For basal markers, CK 5/6, CK 14 and SMA, positivity was defined as detection of any invasive malignant cells positive.
Statistical analysis.
The primary end point was DFS, defined as the time between the date of randomization and the date of the last follow-up or the date of the first event: locoregional recurrence, distant metastasis, contralateral breast tumor or death.
Association between the immunoreactivity with the various antibodies used and different clinicopathological parameters was evaluated by chi-squared test. A p-value of <0.05 was considered to reflect a significant correlation. Survival curves were calculated by the Kaplan-Meier method. The differences between survivals were estimated using the log rank test. Multivariate Cox regression analysis was used to evaluate any independent prognostic effect of the variables on DFS. Two-sided P values of P < 0.05, and of <0.01 for the interaction tests, were considered statistically significant. All analyses were carried out using SPSS 11.0 statistical package.
Results
Patient’s characteristic and clinical outcomes
Complete clinical follow-up information was available for 141 patients. The median follow-up was 52 months (range 1-103 months). During this period, 13.8% patients died from breast cancer and 27.7% patients relapsed. Fifty-six patients were postmenopausal, and at the time of the primary diagnosis 10.4% had lymph node negative disease. 50.8% patients received standard chemotherapy, 7.7% Trastuzumab, 62.3% radiotherapy and 61% patients received hormonotherapy. Other patients characteristic can be seen on tables (1-2-3-4-5) and figures (1-2)
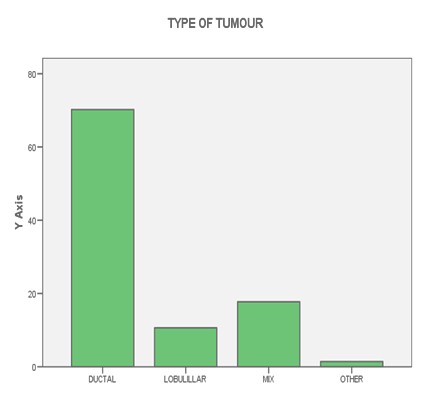
TABLE 1: Histology and phenotype
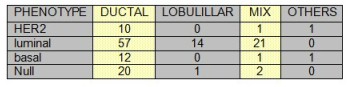
TABLE 2: Number of lymph nodes and phenotype
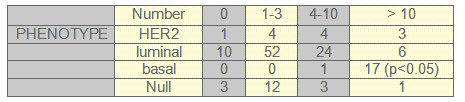
TABLE 3: Menopausic Status and phenotype
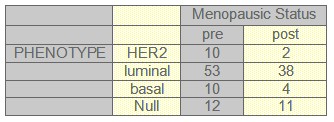
TABLE 4: Radiotherapy and phenotype
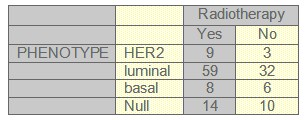
TABLE 5: Histology grade and phenotype
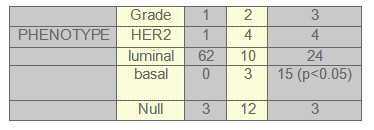
FIGURE 1: LYMPH NODES
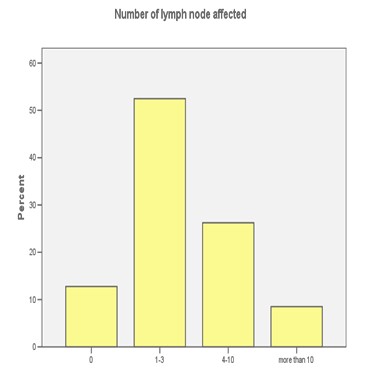
FIGURE 2: HISTOLOGY